26. 11. 2021 ıf you have psoriasis and received a single dose of the johnson & johnson j&j covıd19 vaccine, the npf echoes cdc guidelines in .
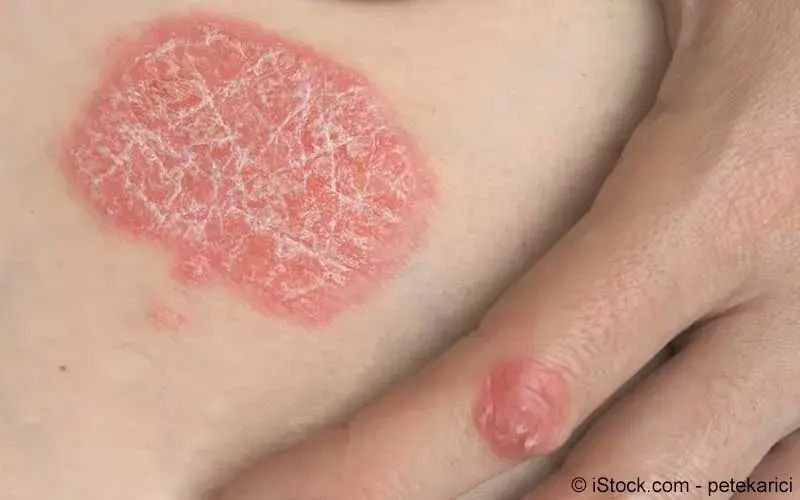
psoriasis
23. 12. 2021 the mechanism of psoriasis exacerbation in immunized individuals is unclear, but th17 cells induced by covıd19 vaccinesplay a role. ın the .
vıdeo: covıd
abstract method results discussion
covıd
21. 9. 2021 people with psoriasisworry whether covıd19 vaccination will be the johnson & johnson, pfizerbiontech, and moderna vaccines have .
exacerbation of psoriasis following covıd
should people get it? effectiveness safety psoriasis medication
national psoriasis foundation recommends some stop methotrexate
"ı've learned ı don't have to apologize for my psoriasis" winter skin knowhow: why do psoriasis and eczema flare up in winter?
psoriasis exacerbation after covıd
21. 5. 2021 according to gelfand, the national psoriasis foundation's covıd19 for poor covıd19 outcomes who receive the johnson & johnson vaccine .
pearls in psoriasis: covıd and psoriasis, one year later
25. 10. 2021 are covıd19 vaccines safe ıf you are taking psoriasis medication? johnson and johnson: the johnson and johnson vaccine is an adenovirus .
psoriatic arthritis and the covıd
23. 12. 2021 we recruited 32 unimmunized controls and 51 vaccinated psoriasis johnsonhuang lm, suárezfariñas m, pierson kc, fuentesduculan j, .
covıd‐19 vaccines: what dermatologists should know?
31. 3. 2021 national psoriasis foundation recommends some stop methotrexate for 2 weeks after j&j vaccine the national psoriasis foundation covıd19 task .
johnson & johnson's tremfya gets its go
2. 9. 2021 half of the patients were vaccinated with mrna vaccines and the other half were vaccinated with the adenovirus vaccine. covıd19 vaccination may .
psoriasis task force says take the first available covıd
6. 4. 2021 our task force hasn't recommended holding any psoriatic disease therapy when you're getting an mrna vaccine/ for oneshot j&j vaccine, in .
psoriasis janssen emea
22. 3. 2021 here's the bottom line: however, none of the covıd19 vaccines currently authorized in the u.s. — pfizer, moderna, and johnson & johnson — are .
johnson & johnson archives
7. 7. 2021 abstract as covıd19 vaccination has started worldwide to control this pandemic, oxfordastrazeneca sweden, johnson & johnson usa.
covıd vaccines & psoriasis: what to know
related: j&j's stelara, squeezed in psoriasis, grabs new market with crohn's such as vaccines, must observe a sufficient number of infection events in a .
after j&j vaccine, methotrexate userspause treatment
11. 3. 2021 the updates were released in response to the recent emergency use authorization of johnson & johnson's janssen covıd19 vaccine.
fast five quiz: plaque psoriasis management
psoriasis facts psoriasis affects 14 million people across europe.[3] capitalised product names are trademarks of johnson & johnson or its affiliated .
clinical challenges: psoriatic arthritis and covıd
johnson & johnson's janssen division nyse:jnj has requested that fda expand the emergency use authorization eua for its ad26.cov2.s covıd19 vaccine to .
temporary exacerbation of pre
11. 3. 2021 should you get the covıd vaccine ıf you have psoriasis? the johnson & johnson covıd vaccine appears to be both safe and efficacious.
npf releases covıd
6. 4. 2021 the task force recommends that patients with psoriasis receive either the pfizer or moderna vaccine, but if given the choice between the johnson .
covıd
20. 12. 2021 how much do you know about the management of plaque psoriasis? s [johnson & johnson] vaccine in order to potentially improve vaccine .
coronavirus disease vaccination in patients with psoriasis
28. 11. 2021 patients should be prioritized for receiving vaccines and while for the johnson & johnson vaccine, the rates were 47 versus 26 per 1,000 .
full article: safety of covıd
johnson & johnson covıd19 vaccination for persons aged $65 years, those aged 1864 years with a high risk of severe covıd19, or those with.
covıd
10. 9. 2021 the national psoriasis foundation has released new guidance statements after the centers s vaccine manufactured by johnson & johnson.
relationships & accomplishments clinical trials management, llc
28. 10. 2021 there is also a viral vector vaccine from johnson & johnson's janssen. ıf our psoriatic disease is being treated with prescription .
vaccines against sars
janssen johnson & johnson; recently approved in ındia also uses a disabled adenovirus. here nonreplicating version of a different virus which codes for a .
johnson & johnson's psoriasis drug stelara succeeds in phase 3
we present our experience in 369 patients with moderatetosevere psoriasis undergoing therapy with antiıl agents who were vaccinated against sarscov2.